Moderna Covid-19 vaccine ‘offers high levels of protection’
Vaccine efficacy against the disease was 94.1%, and vaccine efficacy against severe Covid-19 was 100%, the company reported.

The Moderna coronavirus vaccine may offer very high levels of protection against Covid-19 and there appears to be no evidence efficacy is worse at older ages, primary analysis for the final phase of the study suggests.
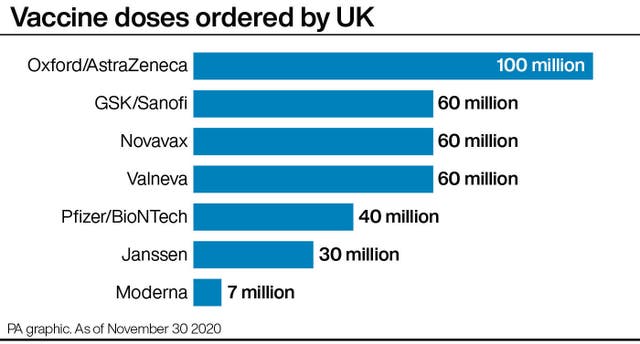
The UK has secured seven million doses of the jab from the US firm – enough for around 3.5 million people in the UK.
Moderna said the analysis of the phase three COVE study of the vaccine candidate, called mRNA-1273, involving 30,000 participants included 196 cases of Covid-19, of which 30 cases were severe.
Vaccine efficacy against the disease was 94.1%, and vaccine efficacy against severe Covid-19 was 100%, the company reported.
It added that the jab is generally well tolerated with no serious safety concerns identified to date.
The study has exceeded two months of median follow-up post-vaccination.
Announcing the results on Monday, Moderna said it plans to request emergency use authorisation from the US Food and Drug Administration (FDA), to apply for a conditional marketing authorisation with the European Medicines Agency (EMA) and to progress with the rolling reviews, which have already been initiated with international regulatory agencies.
Stephane Bancel, chief executive of Moderna, said: “This positive primary analysis confirms the ability of our vaccine to prevent Covid-19 disease with 94.1% efficacy and importantly, the ability to prevent severe Covid-19 disease.
“We believe that our vaccine will provide a new and powerful tool that may change the course of this pandemic and help prevent severe disease, hospitalisations and death.”
The analysis released on Monday was based on 196 cases, of which 185 cases of Covid-19 were observed in the placebo group, versus 11 cases observed in the vaccinated group.
This resulted in a point estimate of vaccine efficacy of 94.1%.
A secondary endpoint analysed severe cases of the virus and included 30 severe cases.
All 30 cases occurred in the placebo group and none in the vaccinated group.
The company said there was one Covid-19-related death in the study to date, which occurred in the placebo group.
Moderna reports that efficacy was consistent across age, race and ethnicity, and gender demographics.
The 196 coronavirus cases included 33 adults aged 65 and over, and 42 participants identifying as being from diverse communities.
Based on prior analysis, the most common adverse reactions included injection site pain, fatigue, myalgia (muscle pain), arthralgia (joint pain), headache, and erythema/redness at the injection site.
The company said these solicited adverse reactions increased in frequency and severity in the vaccinated group after the second dose.
Dr Gillies O’Bryan-Tear, chairman of policy and communications at the Faculty of Pharmaceutical Medicine, said: “Although we await the full details of these results in published form, we can now assume that this vaccine will be approved for use in December.
“Separately, the UK authority the MHRA had announced that they are reviewing the data on an ongoing basis, and it’s likely that approval will also be granted within a fortnight, using emergency authorisation procedures.”
He added: “Although the UK will not receive many doses of this vaccine, realistically it may be enough for 2020 since, separately, the Government has announced it plans to vaccinate one million people per week.
“Moreover, we are expecting similar announcements imminently from both Pfizer/BioNTech – from whom we have secured 50 million doses this year and next – and Oxford AstraZeneca.
“As the vaccine programmes begin to roll out, attention will turn once again to operational issues, but the nation will breathe a sigh of collective relief as the numbers of people vaccinated rolls in.”
Dr Michael Head, senior research fellow in global health at the University of Southampton, said: “These revised findings are very much in line with those previously announced by Moderna.
“This is essentially good news, in that there continues to be a very high level of observed effectiveness, with this effectiveness consistent across older populations and ethnic minorities.
“There were also no serious adverse events caused by the vaccine.
“We must of course reserve a little caution as we await the final published results, but for now we can retain the existing optimism that this new generation of vaccines may be deployed in the near future.”





