What do we know about the Moderna vaccine?
The US company said it is applying for regulatory approval of its Covid-19 vaccine.

US company Moderna said its coronavirus vaccine offers high levels of protection, that trials have shown no serious safety concerns and that it is applying for regulatory approval.
The phase three results suggest vaccine efficacy against the disease was 94.1%, and vaccine efficacy against severe Covid-19 was 100%.
What do we know about the Moderna vaccine?
– How was the trial run and what are the results?
More than 30,000 people in the US took part from a wide range of age groups and ethnic backgrounds.
Two doses were given 28 days apart so researchers could evaluate safety and any reaction to the vaccine.
The analysis was based on 196 cases, of which 185 cases of Covid-19 were observed in the placebo group versus 11 cases observed in the active vaccine group.
Moderna also released data relating to severe cases.
All 30 severe cases occurred in the placebo group and none in the group which had received the vaccine, known currently as mRNA-1273.

– What happens now?
Moderna has applied to US and European regulators for approval.
The company said on Monday that it would request emergency use authorisation from the US Food and Drug Administration (FDA).
It also said that it would apply for conditional marketing authorisation with the European Medicines Agency (EMA) and progress with the rolling reviews, which have already been initiated with international regulatory agencies.
– Will people in the UK get the vaccine?
The UK Government has secured seven million doses of the jab – enough to vaccinate around 3.5 million people.
The vaccine has yet to be approved by the Medicines and Healthcare products Regulatory Agency (MHRA), but doses could begin being delivered next spring if it meets the standards.
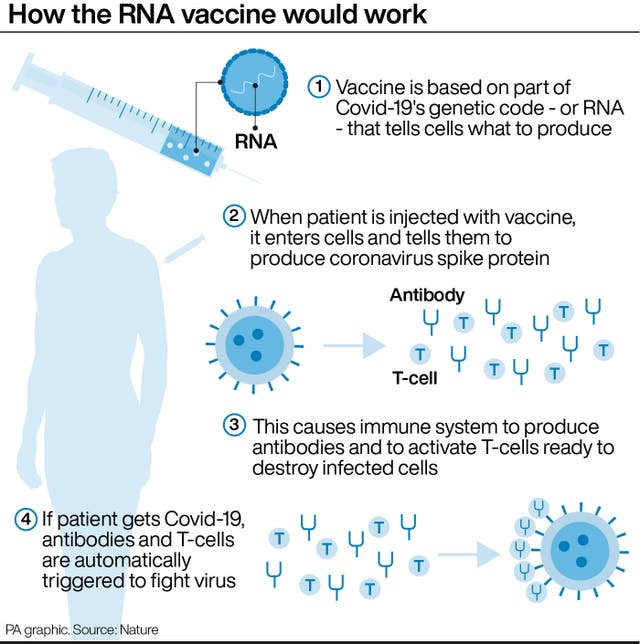
– How does the vaccine work?
The Moderna jab is a messenger RNA (mRNA) vaccine.
Conventional vaccines are produced using weakened forms of the virus but mRNAs use only the virus’s genetic code.
An mRNA vaccine is injected into the body, where it enters cells and tells them to create antigens.
These antigens are recognised by the immune system and prepare it to fight coronavirus.
No actual virus is needed to create an mRNA vaccine. This means the rate at which the vaccine can be produced is accelerated.
– But is the vaccine safe?
Moderna says the vaccine continues to be generally well tolerated, with no serious safety concerns identified to date .
Severe events after the first dose included injection-site pain and after the second dose included fatigue, myalgia (muscle pain), arthralgia (joint pain), headache, other pain and redness at the injection site.
But these were generally short-lived.
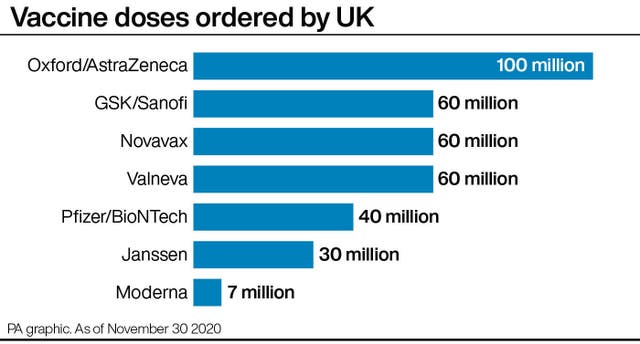
– Is the Moderna vaccine better than those being developed by Pfizer/BioNTech or Oxford and AstraZeneca?
The UK has placed orders for 100 million doses of the Oxford vaccine – enough to vaccinate most of the population – with rollout expected in the coming weeks if the jab is approved by the MHRA.
Oxford data indicates the vaccine has 62% efficacy when one full dose is given followed by another full dose, but when people were given a half dose followed by a full dose at least a month later, its efficacy rose to 90%.
The combined analysis from both dosing regimes resulted in an average efficacy of 70.4%.
The Government also has orders for 40 million doses of the jab from Pfizer and BioNTech, which has been shown to be 95% effective.
However, it is difficult to say whether one option is better than the other, as each trial has had different protocols and they cannot be directly compared.