Third shot of Oxford/AstraZeneca vaccine ‘could be effective booster’
Experts said a study showed that giving people a third dose increased neutralising antibodies against the Alpha, Beta and Delta variants.

A third shot of the Oxford/AstraZeneca vaccine could be an effective booster jab without the need for tweaks, new research suggests.
An Oxford University study found that giving people a third dose more than six months after their second led to a substantial rise in antibodies and increased the body’s T-cell ability to fight coronavirus, including its variants.
Professor Sir Andrew Pollard, head of the Oxford Vaccine Group, said it is not yet known whether people will need a booster shot in the autumn but the new data shows the existing vaccine could be effective.
He said real-world data from Public Health England (PHE) has already shown that two doses offer good protection against hospital admission and death from the Alpha Kent variant and the Delta variant first identified in India.
With two doses currently preventing more than 90% of hospital admissions with Covid, he said it is “difficult to say” whether a third dose could add a few more percent.
But he added: “Boosters are much more about if protection gets lost over time – and we don’t know that – but if it does, could you boost? And the answer to that from these data is yes, you could.
“There’s no indication today that we need boosters, and it is something where we need to keep looking at the data and make decisions as the months go by, about whether that protection that we have is lost.”
He said experts will “expect to see immunity start to wane over time because that does happen” but it will not go back “down to zero”.
He added: “Our immune systems are a bit too clever for us to just look at those numbers… the immune system remembers that we’ve been vaccinated and so, even if we meet the virus some months later, the immune system remembers it, will kick in and make stronger immune responses again and hopefully that will protect most people from severe disease.
“So that’s why I say we just have to watch at the moment to make those decisions (on whether boosters are needed), based on the best evidence as it emerges.”
Teresa Lambe, associate professor at the Jenner Institute at Oxford, said that, regarding antibody responses with a third dose, “we were able to push them up to a level that we saw at the peak of the response after the second dose”.
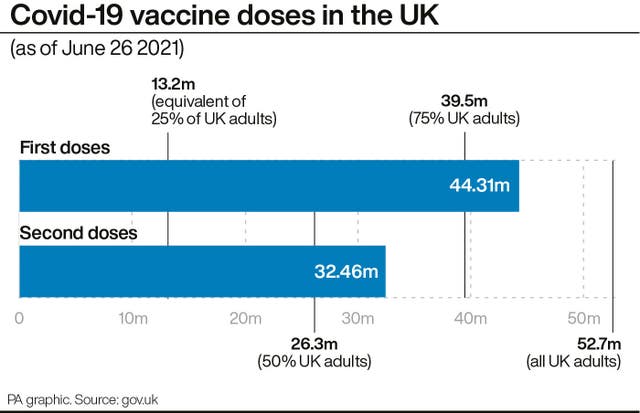
She added: “This is very encouraging because we’ve already demonstrated that two doses of (the vaccine) is both efficacious and effective in the real world.
“We also saw an increase in the neutralising antibodies against a number of variants, so we were able to demonstration increased neutralising antibodies with a third dose against Alpha, Beta and Delta.”
The Beta variant is the one first identified in South Africa that has been worrying scientists.
Prof Lambe added: “Here we show that a third dose of (the vaccine) is well-tolerated and significantly boosts the antibody response. This is very encouraging news, if we find that a third dose is needed.”
Regarding the Beta variant, she said it is difficult to say what “specific level of neutralising antibody is needed” and there is not yet the clarity of data.
In the preprint study, which has yet to be peer-reviewed, some 90 people received a third dose of the vaccine, which was well-tolerated in terms of side-effects.
The study also found that a longer delay of up to 45 weeks between the first and second dose of the Oxford/AstraZeneca vaccine leads to enhanced immune response.
The experts said this is an important finding for countries with limited vaccine supply.
Prof Pollard said the findings should come as “reassuring news to countries with lower supplies of the vaccine, who may be concerned about delays in providing second doses to their populations”.
He added: “There is an excellent response to a second dose, even after a 10-month delay from the first.”
It comes after AstraZeneca and the University of Oxford began new clinical trials on Sunday to test a modified vaccine against the Beta variant.
The booster vaccine trial will involve around 2,250 participants from Britain, South Africa, Brazil and Poland.
The new vaccine has been designed with minor genetic alterations designed to tackle the Beta variant.
Prof Pollard said this study is about staying ahead of the game, so that countries are prepared with booster jabs if they are needed.
He also stressed his belief that countries where adults are still waiting for a first dose should get jabs before boosters are considered here.
He said: “When we have high levels of protection in the UK population, and no evidence of that being lost, to give third doses now while other countries have zero doses is not acceptable.
“We have to make sure that other countries are protected.”





