PM urges people to get Covid jab despite concerns over potential side effects
Concerns have been raised that there could be a potential association between the AstraZeneca Covid-19 vaccine and a rare form of blood clot.

The Prime Minister has urged people to get their Covid-19 vaccine when invited, after concerns were raised about potential side effects of the Oxford/AstraZeneca jab.
Boris Johnson said getting the population vaccinated was “the key thing”.
It comes as a new assessment of the AstraZeneca vaccine is expected to be published later this week.

The European Medicines Agency’s (EMA) safety committee has been reviewing very rare cases of unusual blood clots in people vaccinated with AstraZeneca’s Covid-19 vaccine.
The agency said that the committee has “not yet reached a conclusion and the review is currently ongoing” but it is expected to announce findings on Wednesday or Thursday.
Mr Johnson defended the AstraZeneca Covid-19 vaccine as he visited the pharmaceutical giant’s manufacturing plant in Macclesfield.
“On the Oxford/AstraZeneca vaccine, the best thing people should do is look at what the MHRA say, our independent regulator – that’s why we have them, that’s why they are independent,” he said.
“Their advice to people is to keep going out there, get your jab, get your second jab.”
He added: “The best thing of all is to vaccinate our population, get everybody out getting the jab, that’s the key thing and that’s what I would advocate, number one”.
It comes after reports that a senior EMA official told an Italian newspaper that there appears to be an association with the vaccine and rare blood clots.
Marco Cavaleri, head of vaccines at the EMA, is said to have suggested a clear link, though admitted there was uncertainty how the vaccine would cause the complication.
The EMA has previously said that there is “no evidence” to support restricting the use of the Oxford/AstraZeneca Covid-19 vaccine in any population.
The British regulator – the Medicines and Healthcare products Regulatory Agency (MHRA) – is investigating reports of a very rare and specific type of blood clot in the brain, known as cerebral venous sinus thrombosis (CVST), occurring together with low levels of platelets (thrombocytopenia) following vaccination.
A number of countries have suspended the use of the jab among younger people.
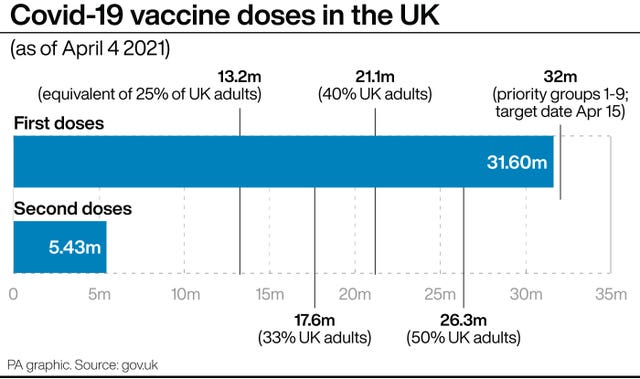
If this were to happen in the UK it could slow the vaccination programme significantly as more than a fifth of the UK’s vaccine haul is tied up in the Oxford/AstraZeneca jab.
The Government has secured a total of 457 million doses, of which 100 million are from AstraZeneca.
But vaccines minister Nadhim Zahawi said he is “confident” that the commitment to offer a jab to all adults by the end of July will be met.
And he said the Moderna vaccine will be rolled out “around the third week of April”.
He told BBC Breakfast: “It will be in deployment around the third week of April in the NHS and we will get more volume in May as well.
“And of course more volume of Pfizer and Oxford/AstraZeneca, and we have got other vaccines. We have got the Janssen – Johnson and Johnson – vaccine coming through as well.
“So I am confident that we will be able to meet our target of mid-April offering the vaccine to all over-50s and then end of July offering the vaccine to all adults.”
Meanwhile he said the MHRA looks “very closely” at reports of adverse reactions to the vaccines.
The agency has said it identified 30 cases of rare blood clot events out of 18.1 million doses of the jab administered up to and including March 24.
There have been seven deaths among the 30 cases.
But the regulator said the benefits of the vaccine in preventing coronavirus outweigh any risks and it urged the public to continue coming forward for the jab.
MHRA chief executive Dr June Raine said: “People should continue to get their vaccine when invited to do so.
“Our thorough and detailed review is ongoing into reports of very rare and specific types of blood clots with low platelets following the Covid-19 vaccine AstraZeneca.
“No decision has yet been made on any regulatory action.”
The 30 cases in the UK include 22 reports of CVST and eight of other thrombosis events with low platelets.
CVST clots stop blood draining from the brain properly.





