Government restates jabs commitment as regulator probes potential side-effects
Concerns have been raised that there could be a potential link between the AstraZeneca Covid-19 vaccine and a rare form of blood clots.

The Government has recommitted to its pledge to offer all adults a Covid-19 vaccination by summer.
It comes as regulators are examining potential links between the Oxford/AstraZeneca jab and rare blood clots.
And reports suggest that the Medicines and Healthcare products Regulatory Agency (MHRA) is considering proposals to restrict the use of the vaccine in younger people.
If confirmed, the rollout of the vaccination programme could be slowed significantly as more than a fifth of the UK’s vaccine haul is tied up in the Oxford/AstraZeneca jab.
The Government has secured a total of 457 million doses, of which 100 million are from AstraZeneca.
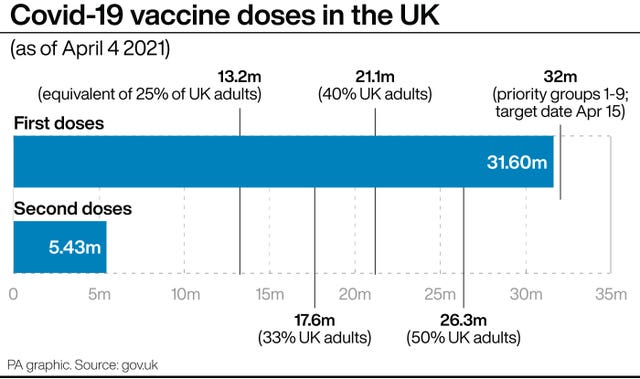
But vaccines minister Nadhim Zahawi said he is “confident” that the commitment to offer a jab to all adults by the end of July will be met.
And he said the Moderna vaccine will be rolled out “around the third week of April”.
He told BBC Breakfast: “It will be in deployment around the third week of April in the NHS and we will get more volume in May as well.
“And of course more volume of Pfizer and Oxford/AstraZeneca, and we have got other vaccines. We have got the Janssen – Johnson and Johnson – vaccine coming through as well.
“So I am confident that we will be able to meet our target of mid-April offering the vaccine to all over-50s and then end of July offering the vaccine to all adults.”
Meanwhile he said the MHRA looks “very closely” at reports of adverse reactions to the vaccines.
The agency has said it identified 30 cases of rare blood clot events out of 18.1 million doses of the jab administered up to and including March 24.
There have been seven deaths among the 30 cases.
But the regulator said the benefits of the vaccine in preventing coronavirus outweigh any risks and it urged the public to continue coming forward for the jab.
Mr Zahawi told BBC Breakfast: “The regulators absolutely look at, very closely, any adverse incidents through the yellow card system.
“And June Raine, who is the chief executive of the MHRA, our independent regulator, said last night that if you get the invite for the vaccine to take that invitation and get the vaccine and get protected.
“At the same time, they are looking at these very rare instances of blood clotting.
“To put it in perspective, we have done almost 20 million vaccinations using the Oxford/AstraZeneca vaccine.
“Both vaccines have saved something like 6,300 lives between December and the end of February, so it’s important to continue to follow what the clinicians, the scientists, the regulators tell us. And we will absolutely do exactly as they say.”
Channel 4 News reported that the MHRA was considering proposals to restrict the use of the Oxford/AstraZeneca vaccine in younger people and a decision could be made imminently.
MHRA chief executive Dr June Raine said: “People should continue to get their vaccine when invited to do so.
“Our thorough and detailed review is ongoing into reports of very rare and specific types of blood clots with low platelets following the Covid-19 vaccine AstraZeneca.
“No decision has yet been made on any regulatory action.”
The 30 cases in the UK include 22 reports of cerebral venous sinus thrombosis (CVST) and eight of other thrombosis events with low platelets.
CVST clots stop blood draining from the brain properly.
But it is not known whether these cases have occurred as a result of the jab, or whether they would have happened naturally in the population anyway.
A number of countries have imposed restrictions on the use of the jab in younger adults.
But the head of the European Medicines Agency (EMA) has said there is “no evidence” to support restricting the use of the AstraZeneca vaccine in any population.
The view is echoed by the World Health Organisation (WHO), which has urged countries to continue using the jab.





