EMA: No evidence to support restricting use of AstraZeneca Covid-19 vaccine
The European regulator said a causal link between unusual blood clots in people who have had the vaccine is “not proven, but is possible”.

There is “no evidence” to support restricting the use of the Oxford/AstraZeneca Covid-19 vaccine in any population, the head of the European Medicines Agency (EMA) has said.
The EMA said a causal link between unusual blood clots in people who have had the vaccine is “not proven, but is possible”, adding that the benefits of the vaccine in preventing Covid-19 outweighed the risks of side effects.
It comes after it emerged Germany was suspending use of the Oxford/AstraZeneca vaccine for people aged under 60 due to fears of a link with rare blood clots.
The EMA said it was meeting on Wednesday in the context of its ongoing review of “very rare cases of unusual blood clots associated with low numbers of platelets” in people who have also had the AstraZeneca vaccine.
The regulator said that at present the review has not identified any specific risk factors, such as age, gender or a previous medical history of clotting disorders, for these “very rare” events.
Speaking at a press briefing, EMA executive director Emer Cooke said: “According to the current scientific knowledge, there is no evidence that would support restricting the use of this vaccine in any population.”
Ms Cooke said 62 cases of cerebral venous sinus thrombosis (CVST) have been reviewed out of 9.2 million people in the European Economic Area (EEA).
The EMA said: “A causal link with the vaccine is not proven, but is possible and further analysis is continuing.
“As communicated on March 18, EMA is of the view that the benefits of the AstraZeneca vaccine in preventing Covid-19, with its associated risk of hospitalisation and death, outweigh the risks of side effects.”
Ms Cooke was asked if a link between the rare cases of blood clots and the vaccine is likely, and she said: “At the moment at this stage of our investigations the link is possible and we cannot say any more than that at this point.”
The EMA said vaccinated people should be aware of “the remote possibility of these very rare types of blood clots occurring”, adding: “If they have symptoms suggestive of clotting problems as described in the product information, they should seek immediate medical attention and inform healthcare professionals of their recent vaccination.”
The EMA said it would provide any further updates during its meeting from April 6-9.
It comes as the German medicines regulator reported 31 cases of a type of rare brain blood clot among the nearly 2.7 million people who received the AstraZeneca jab in the country.
There have been moves in several German regions, including the capital Berlin, to stop using the vaccine in younger people.
Nine of the 31 people suffering clots have died, and all but two of the cases involved women who were aged 20 to 63, Germany’s Paul Ehrlich Institute said. The two men were aged 36 and 57.
Ms Cooke said the 62 figure she mentioned includes a “significant” number of the German cases but not all of them.
The concerns centre on CVST blood clots, which stop blood draining from the brain properly.
Asked if there was a need to look again at AstraZeneca’s vaccine, Communities Secretary Robert Jenrick told Sky News: “No, we don’t, we’re 100% confident in the efficacy of the vaccine, that’s borne out by study after study, by our own independent world-class regulators and by recent research, for example, by Public Health England that’s shown that thousands of people’s lives have been saved since the start of this year alone thanks to our vaccine programme.
“People should continue to go forward, get the vaccine, I certainly will when my time comes, it is a safe vaccine and the UK’s vaccine rollout is saving people’s lives right across the country every day.”
While a definitive link cannot be ruled out, senior regulators have said the benefits of having the vaccine far outweigh any potential risks and have declared it “safe and effective”.
This view is echoed by the World Health Organisation, which has urged countries to continue using the jab.
Covid itself can cause an increased risk of blood clots – a risk that is far higher than any posed by the vaccine.
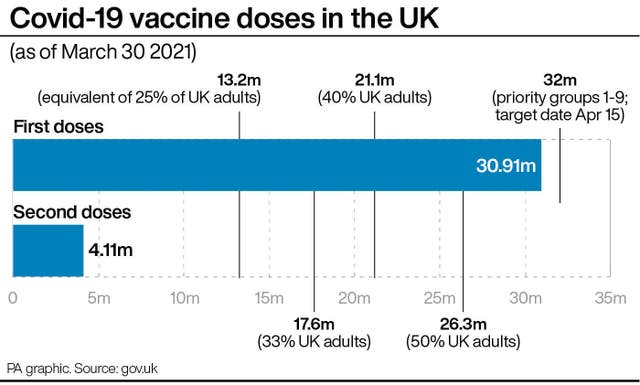
A total of 29,443,531 Covid-19 vaccinations took place in England between December 8 and March 30, according to NHS England data, including first and second doses, which is a rise of 409,891 on the previous day.





